This post includes the following sections:
What is the poultry red mite?
Signs
Mechanisms
Risk factors
Treatment
Importance
Further reading
References
What is the poultry read mite?
Poultry red mite (Dermanyssus gallinae) was first described by De Geer in 1778. It belongs to the sub-class Arachnida. The common name is poultry red mite (PRM) or chicken mite (in the US).
PRM:
• is the most common ectoparasite in poultry
• feeds on blood of the host and, although it favours poultry and other birds, it will also feed on blood from other animals, including humans (Sikes and Chamberlain 1954)
• does not permanently reside on its host, but only feeds there
• spends 30-60 minutes on the hen, during an average visit (Maurer et al. 1988)
• visit the hen for a feeding bout every 2-4 days, generally 5-11 hours after onset of the dark period (at a 12/12h light/dark cycle) (Maurer et al.1988)
Life cycle
PRM has three juvenile stages from egg to adult: larva, protonymph and deutonymph (Figure 1). PRM requires blood from a host for the development of protonymph to deutonymph to the adult stage (Axtell and Arends 1990). PRM also requires blood for adult reproduction. Therefore, during the last three stages, PRM lives as a parasite on poultry, wild birds and sometimes even on humans.
A lifecycle can be completed within 7 to 17 days. A female may lay a total of 30-50 eggs in her life, in clutches of maximum nine eggs. Each clutch is produced after a blood meal.
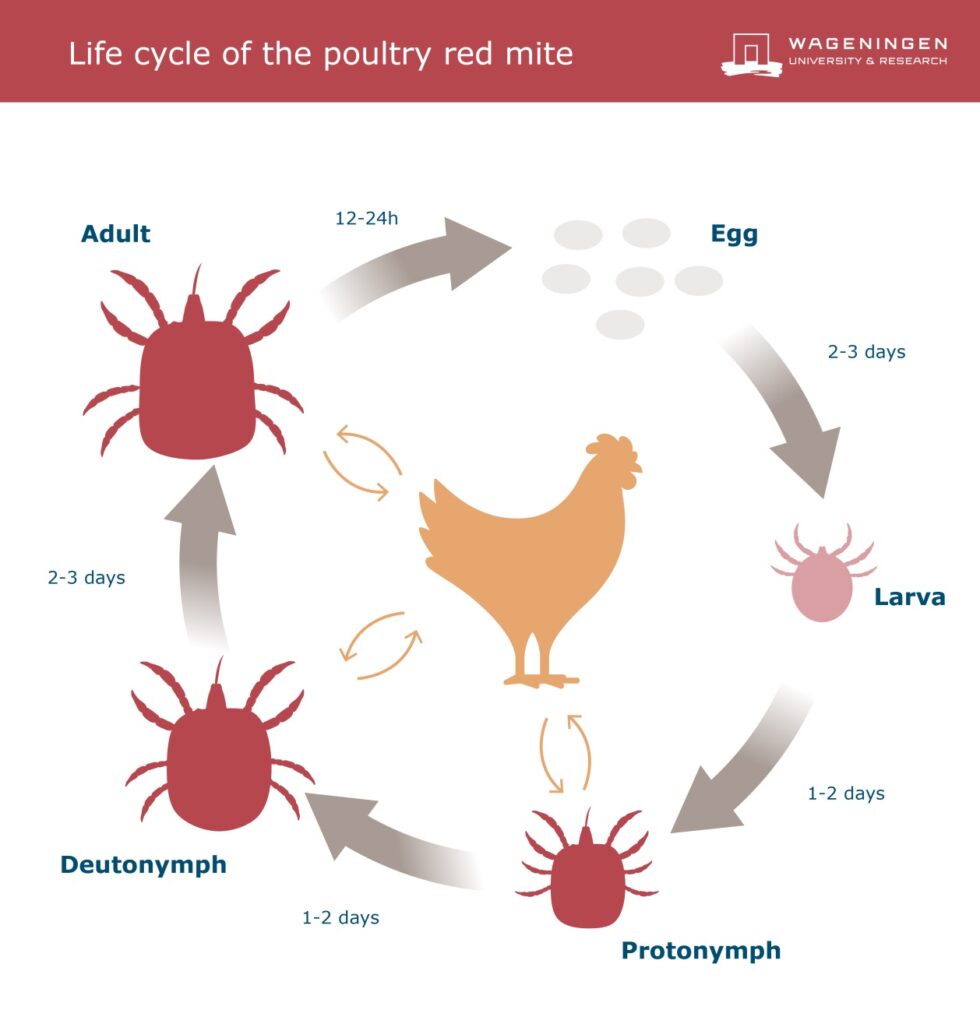
Figure 1 Life-cycle of Poultry Red Mite
.
Signs
PRM infestations have various negative effects on hens, both directly due to their presence on the bird, and indirectly through their blood meals and as a vector for infectious diseases (see Figure 2).
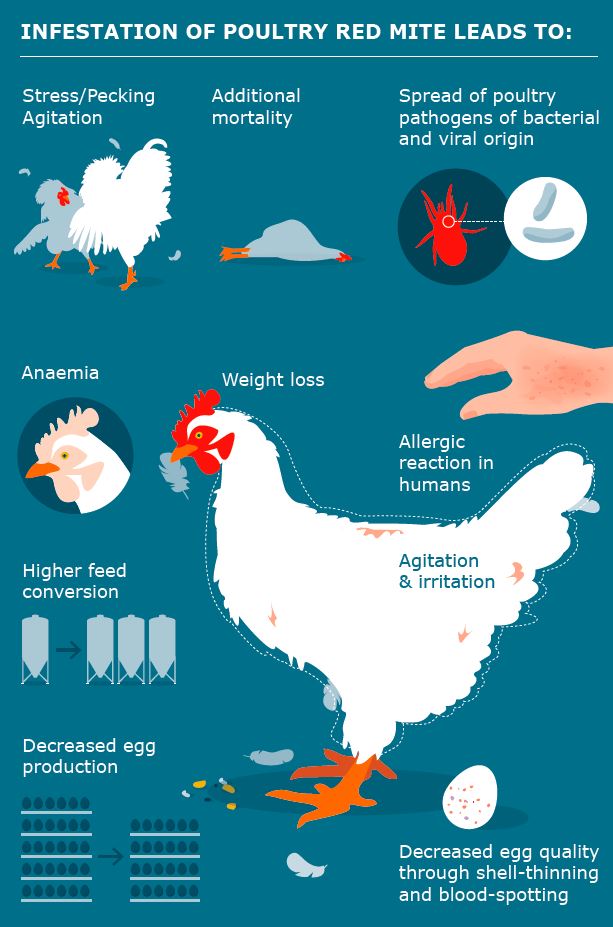
Figure 2 Possible effects of Poultry Red Mite
PRM Infestations in laying hen facilities may cause:
• severe anaemia (Kilpinen et al. 2005)
• high mortality
• stress behaviour (higher levels of preening, head scratching and gentle feather pecking)
• lower body weight
• reduced egg quality due to blood spots (Chauve 1998)
• increased water intake in infested hens
• lower egg production from the flock overall
• increased feed intake and a lower feed conversion ratio in infested hens
• hens avoiding places with high infestations
• general increase of immune response and/or immune suppression of infested hens
• disease transmission by PRM to hens
• reduced feather quality of infested hens
PRM can have a serious impact upon human health causing:
• skin irritation
• itching
• allergic skin reactions (Sahibi et al. 2008; Potenza et al. 2008)
.
Mechanisms
Mite populations can grow fast with availability of:
• the right temperatures (25-35˚C)
• high humidity percentages (70-90%)
• blood from hosts, preferably birds
• hiding places in the vicinity of the hosts resting place
• relative low numbers of poultry red mite predators
For PRM:
• temperatures below -20°C and above 45°C are considered lethal
• sub-optimal conditions reduce the speed of reproduction
• mites are able to survive and reproduce within a wide temperature and RH range
• PRM may survive up to 9 months without feeding under moderate climatic circumstances (5-25°C) (Nordenfors et al. 1999)
• their best survival rate is observed at a RH of 70-90% (Nordenfors et al. 1999)
Hungry mites have several resources that help them find the hen:
• changes in temperature
• changes in odours
• tasting surface skin lipids of the host
• kairomones: these are pheromone-like chemicals, which are thought to play a role in the host location behaviour, but it is unknown what specific chemicals/kairomones are involve.
• carbon dioxide
Once fed, PRM congregate in cracks and crevices to mate. For this, they seem to return to the places where mites have previously congregated, a behaviour that is guided by pheromones (Entrekin and Oliver 1982).
PRM seem to prefer
• cracks smaller than 2 millimetres for breeding and hiding
• hiding places composed of paper, plastic and wood, whereas aluminium and glass are not preferred (Chirico, unpublished data)
PRM can also be found in the manure and litter of heavily infested poultry houses (Maurer et al. 1993).
Population growth of PRM, both post treatment and without treatment, could partly be explained by flock age, hen house and temperature. With this knowledge of the effect of temperature, the PRM population growth can be suppressed with an indoor temperature kept below a farm specific threshold, mostly ranging around 18-20˚C.
.
Risk factors
The most important risk factors for the introduction of PRM into hen houses are:
• introduction of new flocks
• containers and crates
• the farmer and their employees
The most important risk factors for spread of PRM between hen houses are:
• mice, rats and flies
• the feeding system
• shared material and equipment
• the egg conveyer belt
• manure aeration pipes
• removal of cadavers
• visitors and external personnel
• the farmer and their employees
A checklist had been devised for laying hen farmer. This management tool was evaluated by UK and Dutch layer farmers as feasible and useful. The checklist can be found here. A more detailed set of instructions for integrated pest management (IPM) can be found here.
Introduction, and therefore control, of PRM in laying hen facilities is influenced by a suite of all preventive and control measures taken within the egg production chain; i.e. in breeding farms, pullet rearing farms, egg packing stations and transportation routes. Control of PRM should therefore be a collective effort made by all partners within the egg production chain.
.
Treatment
Control of PRM depends largely on synthetic acaricides but is failing because…
• Mites spend most of their lives hidden away in hard to reach cracks and crevices.
• Few active ingredients are available for use, especially when birds are in lay.
• Resistance to available active ingredients is now widespread in European PRM
• Consumers and retailers are increasing their demand for pesticide-free produce
For an (cost)effective treatment it is important to apply a treatment when mite population are relative low. To measure mite populations, monitoring tools are developed. Monitoring a mite population provide insight in the population growth and in the effect of treatment.
In table 1 an overview is given of PRM monitoring methods and devices. Easy and cheap monitoring methods for farmers to monitor the PRM populations are: the tube trap with wooden stick from Van Emous and Ten Napel (2007), the Simplified Passive Trap from Roy et al. (2014) and the Mite Monitoring Score method from Cox et al.(2009).
Table 1 PRM monitoring methods and devices (Reference)
Monitoring method or device Reference
Visual monitoring methods
1. Mite Monitoring Score (MMS) method (Cox et al., 2009)
Traps to show presence of mites
2. MTT-Velcro band mite trap (under development) (Tuovinen et al., 2010)
3. Method for detecting PRM in dust, feathers and impurities (early detection method) (Pavlicevic et al., 2007)
4. Perch trap (Kirkwood, 1963)
Traps collecting mite with visual score
5. Simplified Passive Trap (SPT) (Roy et al., 2014)
6. A tube trap with a wooden stick (Rick Stick) (Van Emous and Ten Napel, 2007)
Traps collecting mite, manual counting with microscope
7. Modified trap after Safrit and Arends (Schulz, 2014)
8. ADAS© Mite Monitor (Anonymous, 2014)
9. Semi Attractive Trap (SAT) (Chiron et al., 2014)
10. Lohmann trap (Mozafar, 2014)
11. Plastic containers with heating pads (Dovc et al., 2016)
12. Paper tube trap (Sokol and Koziatek-Sadlowska, 2016)
13. ADAS© Mite Monitor (Anonymous, 2014)
14. PVC pipe with 13 holes and towel sheet inside (Tucci et al., 1989)
15. Tube containing a fabric or cloth (Maurer et al., 1993)
16. Corrugated cardboard/plastic trap (Nordenfors et al., 1999)
17. A tube trap with corrugated cardboard (Avivet trap) (Lammers et al., 2016)
18 Folded card board (Zenner et al., 2009)
Automated monitoring
19. Automated mite counter (Mul et al., 2015, 2016)
It is easy to make the PVC tube trap with wooden stick. Use 12 cm PVC tube (for electricity) and 10 cm round wood. A small screw is placed in the middle of the length of the round wood to prevent laying hens taking the round wood out of the pvc tube. It is around the screw where you will find the first mites. When scored according to Figure 3 on a weekly base, the mite population can be followed.

Figure 3 PVC tube with wooden stick collecting mites with a visual score classification (Van Emous and Ten Napel, 2004)
Also the Simplified Passive tape Trap (SPT, Roy et al. 2014; Chiron et al. 2014) method is easy to make. Use a 5 – 8 cm long section of 3 cm wide painter’s masking tape and wrap it around cylindrical bars in the poultry system, joining the two ends, but leaving a central space near the bar to serve as a mite refuge (see Figure 4). The number of mites trapped on this sticky refuge should be scored to four different levels: 0 = no mites visible in the trap; 1 = 1-9 mites visible in the trap; 2 = sparse groups of > 10 mites visible in the trap; 3 = clusters of mites visible in the trap.
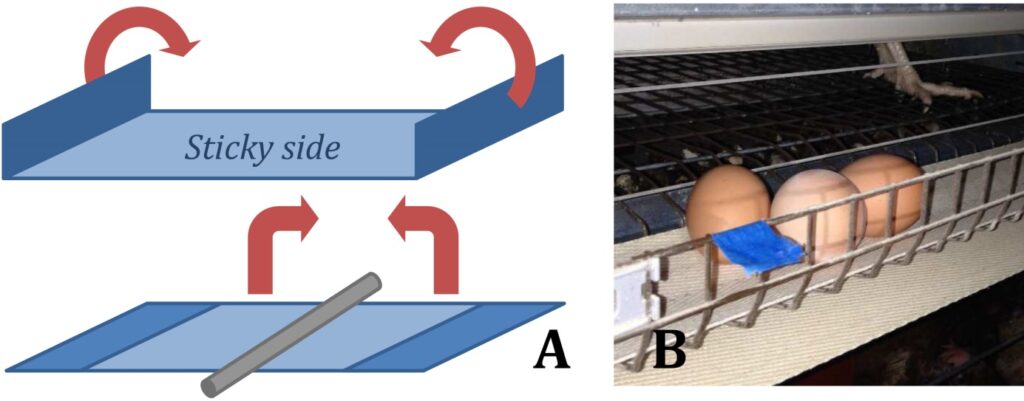
Figure 4 The Simplified Passive Tape trap (SPT) for monitoring D. gallinae is made of Painter’s Masking Tape (panel A) (adapted from Roy et al. 2014). Panel B shows an example of fixation of SPT in an egg laying facility (A.Varescon 2014)
A laying hen farmer has three possibilities to control a PRM infestation;
1. prevent introduction and spread of PRM
2. delay the population development
3. interrupt the lifecycle development by modification of the habitat of PRM (decrease number of hiding places, less optimal temperatures), application of good hygiene measures during the flock and between flocks.
Conventional control methods:
• regular cleaning of poultry facilities
• maintaining good hygiene
• simple cleaning with water, compressed air or by using a vacuum cleaner can remove a large number of mites and eggs (Nordenfors and Höglund 2000) on clean boards and u-shaped profiles
• complete removal of manure once a week. PRM can be found on and under the manure on the manure belt. With the removal of manure, PRM will be removed simultaneously
• keep the housing temperature below 20°C to delay the development of all PRM stages use of acaricides although this may carry the risk of exposing eggs, poultry and humans to their residues (Hamscher et al. 2003). Furthermore, experts indicated that it is only a matter of time before PRM develops resistance to acaricides as pyrethroids, making them ineffective, as already shown in Italy (Marangi et al. 2009), UK (Thind and Ford 2007), Sweden (Nordenfors et al. 2001) and France (Beugnet et al. 1997)
• The use of various types of silica dusts. The main benefit of silica is through its ability to immobilise a mite by adhering to its body, especially to the tarsal part of legs, and preventing locomotion. Silica products are also thought to cause damage to the protective cuticle of PRM, impairing their water balance so that they rapidly dehydrate and die. In humans there is a small risk of silicosis especially during application. Consequently appropriate precautions must be taken. Silica products, especially powdered forms, can cause skin irritations, but other formulations are available (e.g. gel, fluid). The efficacy depends on the quality of the silica, environmental factors and the extent the silica attaches to the treated surfaces. Liquid silica’s seem to be more effective than silica in powder form
• Heat treatment. In between flocks during the empty period the mite population can be effectively reduced by heating hen houses to temperatures above 45°C In Norway, this method was combined with a chemical treatment called phoxime prior to introducing the new flock. In a trial in Norway, all six treated hen houses remained free of PRM during the production cycle after the treatment (Gjevre unpublished data). In the Netherlands, heat treatment without chemical treatment failed to offer similar control and the houses were re-infested within six months (Van Emous personal communication). The main disadvantages of heat-treatment are its high cost and the risk of heat related damage to the hen house equipment. To avoid damage, it is of great importance to continuously measure the temperature and to circulate the hot air with fans to minimise areas with sublethal temperatures where mites could survive.
• Ozone has been used recently.
• Q perch. The Q-perch is a mushroom shaped perch with electrical barriers on the underside enabling to electrocute mites on the route from their hiding places to the laying hen. To date, trials at several test locations show promising results in controling red mite populations (Van deVen, 2016).
Alternative methods
• Natural acaricides include essential oils, herbs or plant extracts which contain a chemical component that kills PRM (George et al. 2008a,b; Maurer et al. 2009). Despite their natural origin, these acaricides may be harmful to humans and animals and may result in residues in the manure. The existing commercial products also lack consistency in the concentration of the actual components due to influences of weather, sun, soil, etc. on the growing plants and due to the variability in concentration of active ingredients in existing commercial products. Furthermore, resistance can build up just as it does with chemical acaricides. Success therefore will depend greatly on the way of application. Participants considered the prospect of success of this measure as only moderate.
• Predatory mites. The use of these predators to control PRM appears promising, especially if the predators will attack all stages of PRM. To withstand and survive the conditions found in the poultry houses, structures are made to provide the predatory mite its preferred habitat.
• Vaccines for PRM in laying hens are being developed as a, suppressive, control method for the mite population in laying hen facilities (McDevitt et al. 2006; Schicht et al. 2014; Bartley 2015).
.
Importance
A questionnaire amongst Dutch laying hen farmers (Van Emous et al. 2005) revealed that farmers estimated the production losses due to PRM populations to be on average € 0,29 per hem per laying round. Farmers estimated the treatment costs against PRM to be on average € 0,14. Nowadays farmers associations indicate that the costs for treatment and production losses are much higher than estimated in 2005, most likely twice as high as estimated.
Based on literature data, Van Emous et al. (2005) was able to estimate the production losses as shown in Table 3.
An questionnaire amongst Dutch laying hen farmers (Van Emous et al. 2005) revealed that farmers estimated the production losses due to PRM populations to be on average € 0,29 per hem per laying round. Farmers estimated the treatment costs against PRM to be on average € 0,14. Nowadays farmers associations indicate that the costs for treatment and production losses are much higher than estimated in 2005, most likely twice as high as estimated.
Based on literature data, Van Emous et al. (2005) was able to estimate the production losses as shown in Table 2.
Table 2 Estimated production losses as a results of different sizes of mite populations
| No mites | Moderate number of mites (mites are not visible during light) | High infestation (mites are visible during light) | Severe infestation (clusters are visible) | |
| Feed intake (grams per hen per day) | 108 | 0 | 1 | 2 |
| Egg weight (grams) | 62 | -0.2 | -0.5 | -1.0 |
| End weight laying hen (grams) | 1800 | -25 | -50 | -100 |
| Second quality eggs (%) | 6 | 2 | 5 | 14 |
| Mortality (%) | 7 | 0 | 1 | 5 |
| Number of eggs per hen (Number @ start of flock) | 345 | 0 | -2 | -10 |
An inventory among most European countries revealed that on average 83% of the poultry farms are infested with poultry red mites (See Figure 5).
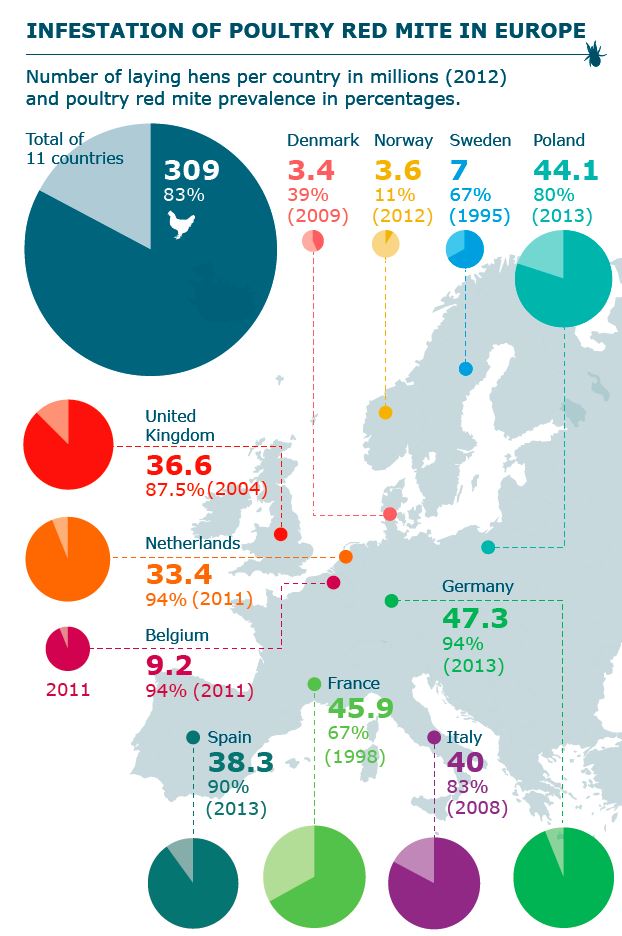
Figure 5 Percentage of with PRM infested farms in EU countries
We conclude that PRM:
• Is the most significant pest of egg laying hens in Europe
• Costed EU egg producers € 130 mill/year in 2004, and probably more today
• May infests up to 100% of flocks in some countries
• Also threatens domestic fowl, companion animals, livestock and humans (allergic reactions)
It is important to realise that PRM can function as a source of infection for new flocks. It is confirmed that Salmonella is able to survive and multiply inside PRM (Valiente Moro et al., 2009), suggesting to eradicate PRM in between flocks during the empty period in order to control Salmonella infections. It is also known that PRM is able to function as vector for several bacteria and diseases as shown in table 3.
Table 3 Pathogens spread by Poultry Red Mite
Table 1. Pathogens spread by Dermanyssus gallinae
| Bacteria | Salmonella spp. P. multocida (fowl cholera) Chlamydia spp Borrelia anserine E. rhusiopathiae Listeria monocytogenes Coxiella Burnetti |
| Viruses | Equine Encephalitus Virus St. Louis Encephalitis (arbovirus) Fowl Pox Avian leucosis Newcastle disease (paramyxovirus) Avian Influenza A |
Further reading
SPECIFIC
Scientific
• Review article on methods to control poultry red mite: Dermanyssus gallinae and poultry production: impact, management, and a predicted compatibility matrix for integrated approaches: (pages 131-141)
• Review article: Sparagano OAE, George DR, Harington DWJ, Giangaspero A (2014b). Significance and control of the poultry red mite, Dermanyssus gallinaer. Annu Rev Entomol 59: 447-466.
English
• Checklist Dermanyssus. 2008
• Factsheet Poultry Red Mite. Mul, M., H. Bens, I. Odink-Schrijver, 2013.
• www.PoultryMed.com
• An article about silica (application)
• Website of Cost action COREMI: Control of Red mites including conference proceedings
• Proceedings of first conference on PRM in Foggia (2015)
• Proceedings of second conference on PRM in Zagreb (2016)
• An elaboration of integrated Pest Management (IPM) for poultry red mite (M. Mul)
Nederlands
• Hulplijst tegen vogelmijt. 2008
• Website over vogelmijt
• Een uitwerking van IPM (integrated Pest Management) voor vogelmijt (bloedluis) (M. Mul)
GENERAL
English
Mozafar F (2014). Tackling red mite in laying hens remains a challenge. World poultry 30 (1): 22-24
Dutch
See references of Dutch publication here.
Van Emous RA, Fiks-van Niekerk TGCM, Mul MF (2006) Bloedluizen (vogelmijten) op papier en in de praktijk. PraktijkRapport pluimvee nr. 17, Animal Sciences Group-Praktijkonderzoek ISSN 15708624 (In Dutch with English summary).
.
References
General
Dutch: see references of Dutch publication here.
Van Emous RA, Fiks-van Niekerk TGCM, Mul MF (2006) Bloedluizen (vogelmijten) op papier en in de praktijk. PraktijkRapport pluimvee nr. 17, Animal Sciences Group-Praktijkonderzoek ISSN 15708624 (In Dutch with English summary)
Specific
English
Anonymous (2014) Website (22-8-2016)
Axtell RC, Arends JJ (1990) Ecology and management of arthropod pests of poultry. Annu Rev Entomol 35: 101-126
Bartley K (2015) Tackling a mitey problem. Vet Rec 177: 38-39
Beugnet F, Chauve C, Gauthey M, Beert L (1997) Resistance of the red poultry mite to pyrethroids in France. Vet Rec 140: 577-579. The resistance of Dermanyssus gallinae to permethrin, and to dichlorvos, was examined on 5 French poultry farms with a history of problems in controlling mite populations and on one farm with no problems. The concentration of permethrin required to kill 50% of the mites on the 5 farms was between 8 and 40 times the concentration required on the control farm. In contrast, no resistance to dichlorvos was detected. This is the first description of resistance to a pyrethroid in D. gallinae in France.
Chauve CM (1998) The poultry red mite Dermanyssus gallinae (De Geer, 1778) : current situation and future prospects for control. Vet Parasitol 79: 239-245
Chiron G, Varescon A, Lubac S, Bicout DJ, Roy L (2014) Méthode d’aide à la prise de décision de traitement contre Dermanyssus gallinae (pou rouge) en élevage de ponte. TeMA 32 :27-34
Cox M, De Baere K, Vervaet E, Zoons J, Fiks-Van Niekerk T (2009) Red mites: monitoring method and treatment. In: Book of Abstracts 8th European Symposium on Poultry Welfare, Cervia, Italy, 18-22 May: 83
Dovc a, Semrov N, Vergles Rataj A, Lindtner Knific R, Nemec M, Trbovsek T, Bradasevec Z, Zorman Rojs O (2016) Evaluation of alternative method for sampling Dermanyssus gallinae (Acari: Dermanyssidae) in poultry farms. In: Final programme and book of abstract of the 2nd COST conference and management committee (MC) meeting, COST Action FA1404 Improving current understanding and research for sustainable control of the poultry red mite Dermanyssus gallinae (COREMI),1st-3rd June, Zagreb, Croatia: 23
Entrekin DL, Oliver JH jr (1982) Aggregation of the chicken mite, Dermanyssus gallinae (Acari: Dermanyssidae). J Med Entomol 19: 671-678. In the laboratory in Georgia, USA, Dermanyssus gallinae (Deg.) was found to aggregate and form clusters of mixed development stages; recently fed mites were found to aggregate more quickly and to form more definite clusters than did unfed ones. This behaviour was caused both by thigmokinesis and by 1 or more pheromones, which were volatile and could be recognised by the mites without direct contact. D. gallinae was attracted little or not at all to several synthetic chemicals that are reported to function as pheromones for other mite species, but it was somewhat attracted to synthetic guanine.
George D, Calaghan K, Guy JH, Sparagano OAE (2008a) Lack of prolonged activity of lavender essential oils as acaricides against the poultry red mite (Dermanyssus gallinae) under laboratory conditions. Res Vet Sci 85(3): 540-542.
George DR, Smith TJ, Sparagano OAE, Gut JH (2008b) The influence of ‘time since last blood meal’ on the toxicity of essential oils to the poultry red mite (Dermanyssus gallinae). Vet Parasitol 155: 333-335
Hamscher G., Priess B., Hartung J, Nogossek MI, Gluender G, Nau H (2003) Determination of propoxur residues in eggs by liquid chromatography-diode array detection after treatment of stocked housing facilities for the poultry red mite (Dermanyssus gallinae). Anal Chim Acta 483: 19-26.
Harrington DWJ, George DR, Guy JH, Sparagano OAE (2011) Opportunities for integrated pest management to control the poultry red mite, Dermanyssus gallinae. World Poult Sci J 67 (1): 83-94
Immediato D, Camarda A, Iatta R, Puttili MR, Ramos RAN, Paola G. Giamgaspero A, Otranto D, Cafarchia C (2015) Laboratory evaluation of a native strain of Beauveria bassiana for controlling Dermanyssus gallinae (De Geer, 1778) (Acari: Dermanysssidae). Vet Parasitol 212 (3/4): 478-482
Kilpinen O, Roepstorff A, Permin A, Nørgaard-Nielsen G, Lawson LG, Simonsen HB (2005) Influence of Dermanyssus gallinae and Ascaridia galli infections on behaviour and health of lying hens (Gallus gallus domesticus). Br Poult Sci 45, 1: 26-34
Kirkwood A (1963) Longevity of the Mites Dermanyssus gallinae and Liponyssus sylviarum. Exp Parasit 14: 358-366
Lammers GA, Bronneberg RGG, Vernooij JCM, Stegeman JA. Experimental validation of the AVIVET trap, a tool to quantitatively monitor the dynamics of Dermanyssus gallinae populations in laying hens. Poult Sci 0:1–10.
Marangi M, Cafiero MA, Capelli G, Camarda A, Sparagano OAE, Giangaspero A (2009) Evaluation of poultry red mite (Dermanyssus gallinae, Acarina: Dermanyssidae) susceptibility to some acaricides in a field population from Italy. Exp Appl Acarol 48: 11-18
Maurer V, Baumgärtner J (1992) Temperature influence on life table statistics of the chicken mite Dermanyssus gallinae (Acari: Dermanyssidae). Exp Appl Acarol 15: 27-40
Maurer V, Bieri M, Fölsch DW (1988) Das Suchverhalten von Dermanyssus gallinae in Hühnerställen. Archiv Geflügelkd 5: 209-215 Dermanyssus gallinae (DeGeer 1778) (red mite of poultry) is a widely spread, economically important ectoparasite in poultry. The purpose of this study was a thorough observation of the host-finding behavior of D. gallinae, in order to prevent a severe increase in the mite population. The most important refuges of D. gallinae are gaps, hollow spaces in the poultry-house installlations, the litter on the floor and the dropping pit. These installations should be constructed without gaps and also be easy to clean. Utilizing an unfoldable, hollow perch, it is possible to detect first appearance of D. gallinae. The mites are not visible in the daytime. Their main activity can be observed at night, approximately 5 hours after total darkness until about two hours before dawn. The estimated duration of the blood meal of D. gallinae is approximately 30 minutes. While searching for a host, the mites tend to spread throughout the poultry-house. D. gallinae reaches the host either by crossing the perch, or even dropping from the ceiling. This knowledge must be observed in the development of preventive measures against D. gallinae.
Maurer V, Baumgärtner J, Bieri M, Fölsch DW (1993) The occurrence of the chicken mite Dermanyssus gallinae in Swiss poultry houses. Mitt Schweiz Entomol Ges 66: 87–97
The occurrence of D. gallinae was investigated in 39 poultry houses of various types and sizes in different regions of Switzerland. Multiple regression techniques were used to search for factors possibly explaining the occurrence of the mites. Chicken mites were found in 85% of the poultry holdings. Hygiene had a large influence on the occurrence of D. gallinae. The densities of D. gallinae were higher in deep-litter systems than in systems where scratching area and dung storing facilities (dung pit or board) were separated. In the 2 batteries under study no D. gallinae were found. There was no difference in the abundance of mites between free-range and indoor systems or between small and big holdings. In the hilly northern pre-alpine region the mites were not as abundant as in the other regions. There were predatory arthropods associated with D. gallinae, but their role could not be assessed with the available data.
Maurer V, Perler E, Heckendorn F (2009) In vitro efficacies of oils, silicas and plant preparations against the poultry red mite Dermanyssus gallinae. Exp Appl Acarol 48: 31-41.
McDevitt R, Nisbet AJ, Huntley JF (2006) Ability of a proteinase inhibitor mixture to kill poultry red mite, Dermanyssus gallinae, in an in vitro feeding system. Vet Parasitol 141: 380-385
Mul M, Van Niekerk T, Chirico J, Maurer V, Kilpinen O, Sparagano O, Thind B, Zoons J, Moore D, Bell B, Gjevre A-G, Chauve C (2009) Control methods for Dermanyssus gallinae in systems for laying hens: results of an international seminar. World Poultry Sci J 6: 589- 600
Mul MF, Van Riel JW, Meerburg BG, Dicke M, George DR, Groot Koerkamp PWG (2015) Validation of an automated mite counter for Dermanyssus gallinae in experimental laying hen cages. Exp. App. Acarol. 66: 589-603
Mul MF, Ploegaert JPM, George DR, Meerburg BG, Dicke M, Groot Koerkamp PWG (2016) Structured design of an automated monitoring tool for pest species. Biosyst Eng 151: 126-140
Nordenfors H, Höglund J (2000) Long term dynamics of Dermanyssus gallinae in relation to mite control measures in aviary systems for layers. Br Poult Sci 41: 533-540
Nordenfors H, Höglund J, Uggla A (1999) Effects of temperature and humidity on oviposition, molting and longevity of Dermanyssus gallinae (Acari: Dermanyssidae). J Med Entomol 36: 68-72 The juvenile development and survival of D. gallinae kept in vitro at different temperatures and humidity were investigated to obtain biological baseline data for a Swedish population. Individual females, eggs, larvae and protonymphs were observed with regard to egg-production, duration of various stages, and longevity when kept at different temperatures and relative humidities. Female mites laid eggs at temperatures between 5 and 45 degrees C with the highest numbers laid at 20 degrees C and RH 70%, but development to larvae and protonymphs was only observed at temperatures ranging from 20 to 25 degrees C. The average duration of oviposition varied from 1.0 to 3.2 days within the temperature range 20-45 degrees C, but was gradually increased to 28 days at 5 degrees C. Specimens survived for up to 9 months without access to food when kept in the temperature range of 5-25 degrees C. Temperatures >45 degrees C and at -20 degrees C were found to be lethal. Longevity was similar for females and protonymphs kept at RH 30% and 45%, but it was enhanced at RH 70% and 90% for protonymphs. This study showed that D. gallinae can survive for a long time without feeding if the microclimate is suitable, but it does not thrive at low relative humidities and at temperature extremes. This indicates that changing of the abiotic conditions in infested poultry houses could be a possible measure to reduce mite populations.
Nordenfors H, Höglund J, Tauson R, Chirico J (2001) Effect of permethrin impregnated plastic strips on Dermanyssus gallinae in loose-housing systems for laying hens. Vet Parasitol 102: 121-131.
Oliveira DGP, Alves LFA, Sosa-Gomez DR (2014) Advances and Perspectives of the use of the entomopathogenic Fungi Beauveria bassiana and Metarhizium anisopliae for the control of arthropod pests in poultry production. Braz J Poult Sci 16 (1):1-12
Pavlicevic A, Pavlovic I, Stajkovic N (2007) Method for early detection of poultry red mite Dermanyssus gallinae (De Geer, 1778). Biotechnol Anim Husb 23 (3-4): 119-127 Dermanyssus gallinae (De Geer, 1778), poultry red mite or chicken mite, is a haematophagous poultry ectoparasite. Mites are usually undetected in poultry flocks with small populations of chicken. The study was conducted to determine the effectiveness of method for early detection of poultry red mite. Investigations were conducted on 13 flocks for a two-year period throughout Serbia and Montenegro. It was observed that method for early detection of chicken mite can improve the efficiency of existing diagnostic methods, that it is simple and decreased the period when parasites were hidden since it enables detection of small number of parasites before the population becomes visible. The method for early detection is recommended to poultry farmers for regular control of the flock and control of the new flock and to veterinarians in poultry production as supplement for diagnostic methods. The sampling of small number of chicken mite, monitoring the movement of the population, effect of the treatment and effect of the disease control should be applied in suspected cases of Dermanyssus infestation to achieve early differential diagnostics.
Roy L, Chiron G, Lubac S, Bicout DJ (2014) Tape-traps as an easy-to-use tool for monitoring and surveillance of the poultry red mite in cage and free-range layer farms. XIVth European Poultry Conference, Stavanger (Norway), June 23-27th 2014
Sahibi H, Sparagano O, Rhalem A (2008) Dermanyssus gallinae: Acari parasite highly aggressive but still ignored in Morocco. In: BSP spring trypanosomiasis, leishmaniasis and malaria meetings. March 30th – April 2nd, Newcastle upon Tyne, 173
Schicht S, Qi W, Poveda L, Struve C (2013) The predicted secretome and transmembrane of the poultry red mite Dermanyssus gallinae. Parasit Vectors 6: 259
Background: The worldwide distributed hematophagous poultry red mite Dermanyssus gallinae (De Geer, 1778) is one of the most important pests of poultry. Even though 35 acaricide compounds are available, control of D. gallinae remains difficult due to acaricide resistances as well as food safety regulations. The current study was carried out to identify putative excretory/secretory (pES) proteins of D. gallinae since these proteins play an important role in the host-parasite interaction and therefore represent potential targets for the development of novel intervention strategies. Additionally, putative transmembrane proteins (pTM) of D. gallinae were analyzed as representatives of this protein group also serve as promising targets for new control strategies. Methods: D. gallinae pES and pTM protein prediction was based on putative protein sequences of whole transcriptome data which was parsed to different bioinformatical servers (SignalP, SecretomeP, TMHMM and TargetP). Subsequently, pES and pTM protein sequences were functionally annotated by different computational tools. Results: Computational analysis of the D. gallinae proteins identified 3,091 pES (5.6%) and 7,361 pTM proteins (13.4%). A significant proportion of pES proteins are considered to be involved in blood feeding and digestion such as salivary proteins, proteases, lipases and carbohydrases. The cysteine proteases cathepsin D and L as well as legumain, enzymes that cleave hemoglobin during blood digestion of the near related ticks, represented 6 of the top-30 BLASTP matches of the poultry red mite’s secretome. Identified pTM proteins may be involved in many important biological processes including cell signaling, transport of membrane-impermeable molecules and cell recognition. Ninjurin-like proteins, whose functions in mites are still unknown, represent the most frequently occurring pTM. Conclusion: The current study is the first providing a mite’s secretome as well as transmembranome and provides valuable insights into D. gallinae pES and pTM proteins operating in different metabolic pathways. Identifying a variety of molecules putatively involved in blood feeding may significantly contribute to the development of new therapeutic targets or vaccines against this poultry pest.
Schulz J (2014) Massnahmen zur bekämphung der roten vogelmilbe (Dermanyssus gallinae) in der ökologischen legehennenhaltung. Dissertation, Freie Universität Berlin. ISBN: 978-3-863877-505-3
Steenberg T, Kilpinen O, Moore D (2006) Fungi for control of the poultry red mite, Dermanyssus gallinae. Proceedings of the international workshop ‘Implementation of biocontrol in practice in temperate regions – present and near future’. DIAS report 119: 71-74
Steenberg T, Kilpinen O (2014) Synergistic interaction between the fungus Beauveria bassiana and desiccant dusts applied against poultry red mites (Dermanyssus gallinae). Exp Appl Acarol 62 (4): 511-524
Sikes RK, Chamberlain RW (1954) Laboratory observations on three species of bird mites. J Parasitol 40: 691-697
Sokol R, Koziatek Sadlowska S (2016) Monitoring the invasion of Dermanyssus gallinae in flocks of layer hens. In: Final programme and Book of abstract of the 2nd COST conference and management committee (MC) meeting, COST Action FA1404 Improving current understanding and research for sustainable control of the poultry red mite Dermanyssus gallinae (COREMI),1st-3rd June, Zagreb, Croatia: 24
Thind BB, Ford HL (2007) Assessment of susceptibility of the poultry red mite Dermanyssus gallinae (Acari: Dermanyssidae) to some acaricides using an adapted filter paper based bioassay. Vet Parasitol 144: 344-348
Tucci EC, Bruno TV, Guimaraes JH (1989) Armadilha para amostragem de Dermanyssus gallinae (Acari Dermanyssidae) em aviários de postura comercial. Arq Inst Biol (São Paulo) Supl, 56: 114
Tuovinen T, Heikkilä P, Juvonen S, Lindqvist B, Tuovinen T (2010) Kanapunkki hallintaan munintakanaloissa. Control of red poultry mite in laying hen houses. Research report MM12-3/312/2010. MTT Kasvintuotannon tutkimus Joikioinen (In Finnish)
Valiente Moro C, Luna CJ de, Tod A, Guy JH, Sparagano OAE, Zenner L (2009) The poultry red mite (Dermanyssus gallinae): a potential vector of pathogenic agents. Exp Appl Acarol 48: 93-104
Van de Ven D (2016) Q-perch, electronic control of red mite. In: Final programme and Book of abstract of the 2nd COST conference and management committee (MC) meeting, COST Action FA1404 Improving current understanding and research for sustainable control of the poultry red mite Dermanyssus gallinae (COREMI),1st-3rd June, Zagreb, Croatia: 21
Zenner L, Bon G, Chauve C, Nemoz C, Lubac S (2009) Monitoring of Dermanyssus gallinae in free-range poultry farms. Exp Appl Acarol 48: 157-166
Zoons J (2004) The effect of light programs on red mite (Dermanyssus gallinae) in battery cage housing, in: Perry GC (Ed.) Welfare of the Laying Hen, p. 416 (CABI).
Dutch
Van Emous RA, Napel J ten (2007) Buis met stokje zeer geschikt voor bewustwording. De Pluimveehouderij 37: 8-9 (In Dutch).





























































 Noodmaatregelen tegen pikkerij
Noodmaatregelen tegen pikkerij Van kuiken tot kip
Van kuiken tot kip